Alzheimer’s disease (AD), a progressive neurodegenerative disorder, is the leading cause of dementia globally, affecting over 32 million people as of 2023. Characterized by cognitive decline, memory deficits, and behavioral changes, AD is marked by the accumulation of amyloid-β plaques and neurofibrillary tangles in the brain’s gray matter. Aging is a significant risk factor, particularly in the prefrontal cortex (PFC), a region critical for higher cognitive functions. Despite years of research, the molecular mechanisms distinguishing AD from normal aging remain elusive. In a recently published study titled “Stereo-seq of the prefrontal cortex in aging and Alzheimer’s disease,” researchers at Tulane University in the Deming Department of Medicine performed the first subcellular-resolution spatial transcriptome atlas of the PFC from Alzheimer’s disease patients. The researchers published their findings in the January 8th, 2025, issue of Nature Communications.
Background and Objectives
The researchers aimed to uncover the molecular mechanisms underlying aging-related susceptibility to AD by comparing transcriptomes from the PFC of six male AD patients and six age-matched controls. Previous studies, such as those using 10X Visium, revealed AD-associated gene expression changes but lacked the spatial resolution to dissect single-cell interactions. By employing Stereo-seq, this study sought to:
- Identify transcriptional differences across PFC layers.
- Characterize cell-cell interactions influencing AD pathology.
- Highlight potential therapeutic targets.
Methodology
Using Stereo-seq, the team analyzed cryosections of PFC tissue, focusing on seven predominant cell types: astrocytes (Ast), excitatory neurons (Ex), inhibitory neurons (Inh), microglia (Mic), endothelial cells (End), oligodendrocyte progenitor cells (Opc), and oligodendrocytes (Oli). By aligning transcriptional data with spatial maps, they generated a detailed atlas revealing layer-specific and cell-type-specific alterations in AD.
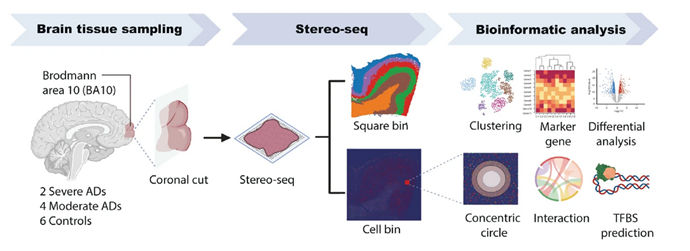
From Gong Y. et al. Nature Communications 2025
Key Findings
- Disruption of Laminar Structure The study identified significant structural changes in PFC layers, particularly in advanced AD cases. Layers II-VI showed marked thinning and transcriptional disruptions, highlighting the vulnerability of these regions to neurodegeneration.
- Gene Modules Linked to Neuroprotection
- Immune Response and Inflammation Regulation: Genes regulating immune activity were upregulated in stressed neurons and glial cells, emphasizing the role of inflammation in AD progression.
- Protein Homeostasis: Modules governing protein degradation—crucial for clearing amyloid-β and tau aggregates—were significantly downregulated.
- Synaptic Function: Genes involved in synaptic transmission and plasticity showed decreased expression, correlating with cognitive decline in AD.
- ZNF460: A Potential Therapeutic Target The transcription factor ZNF460 was identified as a regulator of the aforementioned gene modules. Its downregulation in AD suggests it could be a promising therapeutic target for restoring neuroprotective pathways.
- Spatial Patterns of Stress Response Stressed neurons exhibited elevated mitochondrial gene expression, correlating with neuroinflammation and oxidative stress. Adjacent glial cells—particularly astrocytes—showed transcriptional changes supporting neuronal survival and amyloid-β clearance.
- Altered Cell-Cell Communication Communication networks between cortical layers, mediated by ligand-receptor interactions, deteriorated with AD progression. Notably, the loss of glutamate signaling in excitatory neurons underscored synaptic dysfunction as a hallmark of AD.
Implications for Alzheimer’s Research
This study provides critical insights into the spatial and molecular dynamics of AD in the PFC:
- High-Resolution Mapping: By identifying transcriptional changes at single-cell resolution, researchers can pinpoint early markers of AD pathology.
- Targeted Therapies: Discovering regulators like ZNF460 opens avenues for precision medicine aimed at restoring neuronal health.
- Enhanced Understanding of Disease Progression: The spatial context of gene expression reveals how cellular interactions evolve in response to AD pathology, offering a more holistic view of the disease.
Expanding Access to Stereo-seq Technology
The accessibility of Stereo-seq services is crucial for advancing research. MiRXES, a leading genomics and bioinformatics service provider, was the first independent U.S.-based organization to offer Stereo-seq. In January 2025, MiRXES expanded its capabilities to include formalin-fixed, paraffin-embedded (FFPE) tissue, a common medium used for archived samples. This advancement significantly broadens the scope of AD research by enabling the study of historical tissue collections.
Conclusion
Integrating Stereo-seq into Alzheimer’s research marks a new era of discovery, offering unprecedented clarity into the disease’s cellular and spatial dynamics. By bridging the gap between molecular profiling and spatial context, this technology holds the potential to unravel the complexities of neurodegeneration and pave the way for innovative therapies. As accessibility to Stereo-seq services grows, the scientific community stands on the cusp of breakthroughs that could redefine our understanding and treatment of Alzheimer’s disease.
Keywords and abbreviations: Stereo-seq, spatial transcriptomics, Alzheimer’s Disease (AD), prefrontal Cortex (PFC), neurodegeneration, astrocytes (Ast), excitatory neurons (Ex), inhibitory neurons (Inh), microglia (Mic), endothelial cells (End), oligodendrocyte progenitor cells (Opc), oligodendrocytes (Oli), formalin-fixed paraffin-embedded (FFPE) tissue, Visium, dementia, genomics


